chart 1
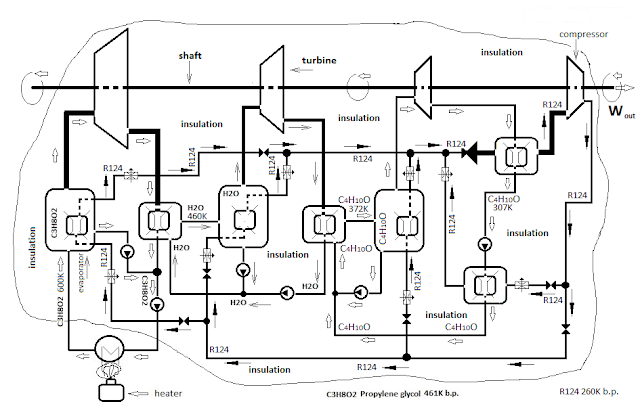
chart 2
chart 3
chart 4
Charts 3 and 4 am figuring engines of the same capacity of the converters of heat into mechanical energy as chart 1 and 2, but here I have no imaginary "free" cooler. Close the cycle by using force to close the cycle - cooling gas as I use cooler - External combustion - internal cooling engines. Waste heat (20%) cooler return it in the evaporators of the unit. Accept that besides waste heat in evaporators returns heat equal to the force I used to close the cycle + 20% of heat . So aggregates of diagrams 3 and 4 for the same capacity of the turbines will produce 40% less mechanical energy than units with imaginary "free cooling". If a cooler is free - 100kW, and if cooling is "paid" - 60 kW of power.
External combustion 100kW
External combustion - internal cooling - 60kW
for the same capacity of the turbine (piston / cylinder)
chart 5
chart 6
On chart 5 am drawn external combustion engine with real free cooler - environment. On chart 6 - engine by method - external combustion - internal cooling, with the same parameters.
Assume - on 20% waste heat - 100kW power of external combustion engine
Verdict:
1.On 20% waste heat - 100kW useful power of external combustion engine
On 20% waste heat - 60kW useful power of external combustion - internal cooling engine for one and the same capacity of the turbines.
2.For the same capacity of the converters of heat into mechanical energy engine - external combustion - internal cooling gives 40% less mechanical power, but uses 40% less heat - no waste heat.
3. Another small deficiency on engine by external combustion method - necessarily need heater to heat the hot part, so that the engine to have a cold part. On the engine by external combustion - internal cooling method the cold part we create it, so that we can use any heat, including of heater.
chart7
chart 8
Let's
take one external combustion engine as
the chart 2 filled with the same three working substances
(ammonia, R41, R14) and go to Planet X, which has an atmosphere with a
temperature of 130K - chart 7. Now, for such a unit will have free cooling,
as we have in mind that the last working substance (R14) has a boiling point of
145K, and the atmosphere of Planet X on which the temperature is 130K. Light a
burner and heat ammonia to 290K. I accept that for a 20% waste heat engine will
gives 100kW mechanical energy.
Redesign
the External combustion engine to an External
combustion- internal cooling by removing heat exchanger which cools the last
working substance to liquefy in the atmosphere of the planet X - chart 8. Set in its
place cooler loaded with nitrogen. Waste heat set it back into evaporators. As
I said above, now I lose 40% of the power output of the unit, but also
decreased 40% fuel in the burner.
On Planet X unit working with these substances can only work with heater, whether
external combustion engine or an external combustion - internal cooling engine.
On
Earth we do not need a heater - Sun heated the atmosphere at 290K and hence
heat ammonia. But on Earth mandatory for these working substances unit must be performed by External combustion - Internal cooling method, because no natural cold part.
Conclusions:
Engine
- external combustion
1
With external combustion engine ever we need a heater.
2
In these units have free cooling
3.
We have waste heat
Engine
- External combustion- internal cooling
1 Heater is not mandatory
2
For the same capacity of converters of heat into mechanical energy
(turbines; pistons / cylinders) has a lower power than external combustion
engine
3
No waste heat
Summary
of advantages and disadvantages of the method for converting heat into
mechanical energy - External combustion - internal cooling
When
using External combustion engine we pay for heating and cooling is free.
When
using External combustion - internal
cooling engine is not required to pay for heating, but must pay for cooling.
06 January 2016
I made some mistakes in the calculations for external combustion - internal cooling. True comparison between the two engines in the same capacity of the converters of heat into mechanical energy should look like:
At 100 kW useful power and 20 kW waste heat
1. External combustion engine:
120 kW power of the heater
100 kW useful power
20 kW waste heat
2. External combustion - internal cooling engine:
80 kW power from the heater
80 kW useful force
0 kW waste heat
20 kW from the gross mechanical power is converted into heat (so the net power becomes: 100-20 = 80 kW)
+ 20 kW waste heat - these two amounts of heat returned to the evaporator(s). This requires to reduce the power output of the heater wiht 40 kW : 120 - (20 + 20) = 80 kW
It would be good to think about things in depth before presenting them to readers. I beg your pardon!
:) :) For my next invention I intend to never wrong :) :)
06 Jan 2016
Svetozar the Cold