External combustion- internal cooling engine with two working substances on pistons§cylinders
(Rankin cycle and zero cycle on pistons)
I will present some reflections on the use of two
working substances (with different boiling points) who work in thermally
isolated environment by pistons. I will discuss several phases of performing a work of the substances and phase of application of force on one of them, and next
week will try to connect them in a "analog" type who will represent
external combustion- internal cooling engine of two working substances filled
with pistons .
Let us have two Dewar containers with two working
substances in liquid state. One with a high boiling point will call it Alpha, and the other with a low boiling point will call it Beta substance. The
containers are connected to the cylinders in which the pistons move.
All processes of course developed in thermal
insulated environment.
Let in one Dewar have some amount of liquid substance
Alpha with a temperature higher than its boiling point -T1 on diagram 1.
The container is
connected to the cylinder and piston position A on diagram1. We put weight N kg.
the piston for opening the valve substance will expand (evaporate) and pushed
the piston - respectively the weight of a distance -position B. Let
equilibrium between the pressure in the container and the weight on the piston
is in such an increase in volume, wherein the substance cools down to a
temperature T2 = (T1 - Tbp) / 2,where Tbp is a boiling point of the substance.
In another Dewar we have some amount of liquid
substance Beta at temperature T1 which is the initial temperature of Alpha.
Container is also connected to the cylinder/piston - chart 2.
We take gas from
cylinders of the substance Alpha and put them in a heat exchanger to a
container of the substance Beta. On the piston put weight equal to ½ of weight N where Alpha is in equilibrium at T2. Let the amount of the substance Beta be
such that upon opening of the valve together with the gases of Alpha gravity
move the same distance, and the system goes into equilibrium at a temperature
T3 equal to the boiling point of the Alpha - position B in diagram 2. In its
equilibrium position volume on Beta has been extended so that the temperature of
the two substances (beta has a low boiling point and heat exchange between
them) is equal to the boiling point of the Alpha - gases Alpha liquefies at
equilibrium of the gas pressure of the Beta and weight equal to to ½ of gravity
N.
Now I want to discuss the question - What is the smallest
weight that if we put on the piston to return it to the starting position - to
return to the starting position parameters of volume, pressure and temperature
of the substances in these processes of charts 1 and 2? By low conservation of
energy this will be another added weight Nkg for Alpha, and 1 / 2Nkg for Beta - chart 1a for Alpha,
and charts 2a and 2b for Beta.
As work has made the substance, so the force
applied to it to perform the same work on it, and the substance returns to its initial values of temperature, volume and pressure .
Let pistons of the two containers with different
substances are connected to the "scale" - diagram 3a,
or better of the
crankshaft in the opposite direction of movement - diagram 3c.
In the
condition of opening the valve Alpha will be in equilibrium with the weight Nkg. and Beta by weight 1 / 2Nkg i.e. the piston of Alpha acting force twice larger than
the force on the piston Beta. Alpha substance has power precisely so as to
return the substance beta to its initial state after opening the valves (as I
follow the logic of the previous charts 1a, 2a and 2b) - diagrams 3b; 3d
3d.
The
temperature of Alpha in the container and cylinder (liquids and gases) in equilibrium position by default (position b) T2 = (T1 -Tbp) / 2, and the
temperature of Beta in position b (its initial state) is T1, respectively
liquid Alpha which heat exchange with Beta also has a temperature T1.
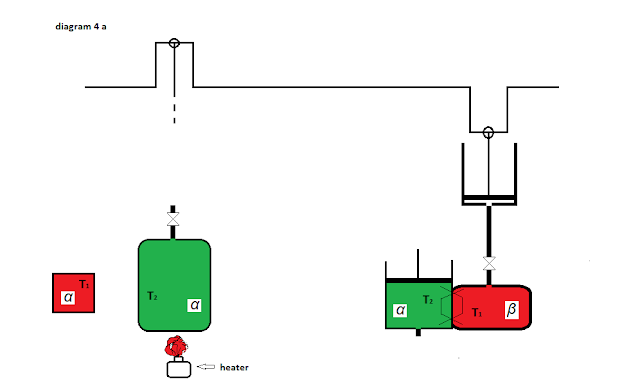
If I remove the
liquid Alpha from heat exchanger , and in its place put gas Alpha from cylinder will return
to the starting position at which gases Alfa at temperature T2 and liquid Beta at temperature T1 perform work as push the piston respectively gravity 1 / 2Nkg to
their equilibrium position as I start - diagrams 4a; 4b; 4c .
To return to the starting position the container
with liquid Alpha substance must be heated liquid alpha in the container of
temperature T2 to temperature T1. This heat has turned into mechanical energy.
4c start (end) position
Discussed above processes and actions with both
substances Alpha and Beta them harnessed in one unit to perform work on behalf
of a heat source. Naturally as with any patterns external combustion -
internal cooling engine heat source can be the environment - Alpha substance must must be a
boiling point lower than ambient temperature. These few several phases of
action I arrange them in a station that end (or initial) phase performs some work (raising the weight 1 / 2 Nkg ,of gases on substance Alpha, and substance Beta ,where the Nkg it is the strength of the alpha) on account of the heat source. I summarized :
Based on pre-set temperatures, quantities and volumes of two working
substances can receive mechanical force as one substance - Alpha gets heat from source and works in Rankin cycle, and other Beta participate in the closing
cycle on Alpha, and in start / end point its parameters remain unchanged ( Zero cycle).
Let's Alpha be ammonia ( 240K bp, 196K mp) and Beta is nitrogen (77K bp). Let quantities Alpha and Beta are such that in the equilibrium position - position B from a temperature of 300K and 270K of nitrogen and ammonia gases temperature decreased to 200K - close to the freezing point of the ammonia,due to the increase a volume on Beta . Now when I open the valve will have two forces - a piston which rises weight 1 / 2N kg, and another piston - over ammonia gases, due to contraction of temperature close to freezing point rise weight Xkg. Total work done from position A to position B will proportional on 1/ 2N kg + Xkg. What weight can raise (to N eventually) I do not know. I would prefer to check it empirically :)
11.03.2016
Another more close to our ideas example of a puzzle with the force of contraction - diagram 5a
To be continued
12.03.2016
Here are the reviewed processes in unit - two working substances on pistons - diagram 6
indications:
1 - double-acting piston
2 - evaporator
3 - compression container
4 - valve
5 - reducer valve
6 - heat exchanger with the heat source
7 - pump
8 - heat exchanger
Good external combustion - internal cooling engine must have a good thermal insulation. In the case shall the pistons and cylinders must to be of materials with low thermal conductivity.














Няма коментари:
Публикуване на коментар